A Radioactive Breakthrough

Radioactivity, one of the most transformative phenomena in modern physics, radically altered our understanding of atomic structure and energy. Marked by serendipitous observations, its discovery ushered in an entirely new era in science, laying the foundation for fields as diverse as nuclear physics, radiology, and energy production. The history of radioactivity, punctuated by the efforts of several key figures, centers on a handful of groundbreaking discoveries in the late 19th and early 20th centuries, beginning with Henri Becquerel’s unexpected results in 1896.
Significant progress had already been made in understanding the basic structure of matter at that time. While the existence of the atom was confirmed, it was thought to be an indivisible, stable unit of matter, according to the atomic theory proposed by John Dalton in the early 19th century. Since the understanding of atoms was still incomplete, the idea of discovering forces or particles beyond the atom’s structure seemed far-fetched and unlikely. This notion pictured a stable universe and unchanging matter. But hidden within the heart of certain elements, atoms were quietly revealing that they were far from stable.
A French physicist, Henri Becquerel, was studying substances that glow after being exposed to light—phosphorescent minerals—when he stumbled upon something far more mysterious. He had been studying uranium salts, which were believed to emit some form of radiation when exposed to light. However, when he stored the salts in a dark drawer wrapped in opaque paper, the photographic plates he used to capture radiation still became fogged. It was as if the uranium salts were emitting some kind of invisible energy, not tied to any external light source.
Becquerel had revealed a profound truth: certain elements could spontaneously emit energy, and this energy came from the very nucleus of the atom, not from any external source. The concept of radioactivity needed further investigation, and this was where Marie and Pierre Curie stepped onto the forefront. The Curies started working in the laboratory at the Sorbonne in Paris, inspired by Becquerel's findings and sought to investigate the nature of these peculiar rays in greater depth. In 1898, the Curies discovered that thorium also emitted radiation, but it was the discovery of a new element that would forever change the course of history.
They discovered a previously unknown element within the mineral pitchblende (now known as uraninite), which was coined as radium. The far more intense radiation released by the element allowed the Curies to demonstrate that the radiation emanated from the atom itself, rather than being influenced by sunlight. During their investigation, another element was identified, polonium, named after Marie Curie’s native country of Poland. This work from the Curies proved to be hugely instrumental in changing the current perception of the atom and its stability. The fact that the atom decayed itself to become stable paved the way for a better understanding of nuclear structure and behavior. Marie Curie’s contributions were unparalleled. The novel methods developed by her for isolating and measuring radioactive substances laid the foundation for future advances in nuclear science.
The Curies did not, however, yet understand the deeply dangerous implication of their work as the hazardous effect of radiation on the human body had not yet been fully realized. Decades-long exposure to highly radioactive elements like uranium, radium, and polonium led to the tragic death of Marie Curie in 1934 due to aplastic anemia. The chair she used to sit on while examining elements in her office along with the door she used to open with her radioactive hands still sends GM counters beeping continuously due to the radiation they let out.
Despite the personal toll, the Curies’ work paved the way for other geniuses to further investigate radioactivity. Among them was Ernest Rutherford, often known as the father of nuclear physics. In 1899, together with his colleague Frederick Soddy, he identified alpha and beta radiation. Alpha radiation consisted of helium nuclei, while beta radiation was composed of high-energy electrons. Later in 1911, Rutherford conducted his most famous work, the gold foil experiment. The practical included firing high-energy alpha particles through a gold foil about 1000 atoms thick onto a fluorescent zinc sulfide screen. To everyone’s bewilderment, most of the alpha particles passed straight through. This suggested that the atom was mostly empty space with a minute positive charge concentrated in the center.
With the discovery of radioactivity and the subsequent advancements in nuclear physics, the study of atoms took on new dimensions. The concept of half-life—the time required for half of the atoms in a sample of a radioactive isotope to decay—was developed by Frederick Soddy and became a vital tool for understanding the behavior of radioactive materials. As the study of radioactivity expanded, it also opened new doors in science and technology. The development of nuclear energy during the mid-20th century, though controversial and dangerous at times, was a direct offshoot of discoveries in radioactivity. Nuclear power plants harness the energy released during nuclear fission, which is related to the processes of radioactive decay.
Similar Post You May Like
-
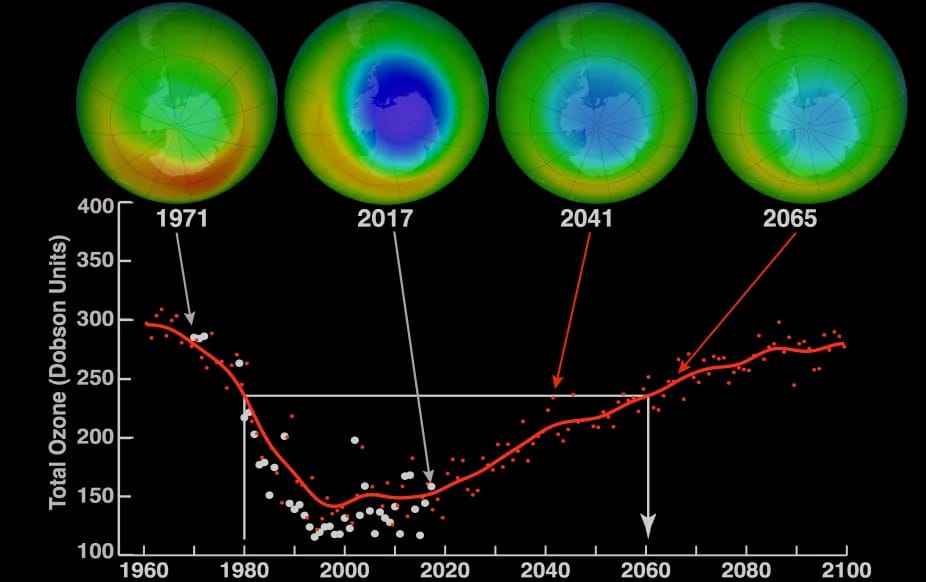
CFCs, HFCs and their long, troubled history
At its peak, the ozone hole covered an area 7 times larger than the size of Europe, around 29.9 million km2, and was rapidly expanding
-

The Origin of Universe: Deciding point where it all began!
Let us unravel and surf through the ideas throughout ages to understand what the universe and its origin itself was to its inhabitants across history.
-

The Artemis Program
Inspired by the Greek goddess of the Moon, twin sister to Apollo, the artimis program was named on 14 May 2019 by Jim Bridenstine.