Chlorofluorocarbons (CFCs), Hydrofluorocarbons (HFC), and their long, troubled history
CFCs are organic compounds containing carbon, fluorine, and chlorine as indicated by their name. They have a long history of use as propellants, as agents in packing materials and refrigerants (for example, freon).
While CFCs were invented in the 1890s by Fredric Swarts they were not used at a commercial scale until the 1900s. Before the mass adoption of CFCs in the 1920s, refrigerators used incredibly toxic gases such as ammonia(NH3), Methyl-Chloride(CH3Cl), and sulfur dioxide(SO2) as refrigerants. Even a small leak in a refrigerator unit could prove deadly and there were a number of fatal accidents in the early 1900s due to methyl chloride leaks. To solve this problem there was military-sponsored experimentation with Carbon and Chlorine compounds such as CCl4 which was used in fire extinguishers before and during WW1. However, it was not until Thomas Midgley Junior, of General Motors discovered Freon, a CFC, which was a non-toxic alternative to the refrigerants of the day. CFCs were finally adopted on a commercial scale. They are safe for humans, in fact after the World War 2 they were as propellants used in most kinds of sprays. They were even used in asthma inhalers as propellants.
However, what was not known until more than 40 years later was the environmental impact of CFCs, specifically their impact on ozone (O3). Ozone is an inorganic molecule consisting of only 3 oxygen atoms and plays a vital role in inhibiting the level of UV rays originating from the Sun that hit the surface of the earth. Excessive UV ray exposure is implicated in an increased risk of melanoma and a plethora of other dermatological conditions from skin burns to wrinkling and hyperpigmentation. In 1957 an independent scientist named James Lovelock Invented the electron capture detector which is a device that is utilized in gas chromatography to detect trace compounds in a gas sample. Dr. Lovelock utilized the device to detect a specific kind of CFC in the atmosphere (trichlorofluoromethane, CFC-11) for the first time over Ireland at 60 parts per trillion (ppt) concentration. After this discovery, Dr.Lovelock went to Antarctica on a self-sponsored expedition to detect CFCs over the south pole and found them to be present in all of the collected air samples. However, after detecting it he came to the incorrect conclusion that CFCs were not harmful to the environment which was the scientific consensus of the time. It was not until late 1973 that the idea of CFCs being incredibly harmful to the environment was floated around in the mainstream. A scientist named Sherry Rowland and his fellow Mario Molina heard Lovelock’s conference on the atmospheric presence of CFCs and his conclusion that they were not harmful, after which both Rowland and Molina wanted to figure out the fate of CFCs in the atmosphere and their work discovered the true nature of CFC’s in the atmosphere. They found that while CFC’s are mostly inert in the lower troposphere, they are prone to become hyper-reactive in the stratosphere as the Sun’s UV rays Cleave the C-Cl bond present In CFC’s Creating a Cl free radical which goes on to react with thousands of Ozone molecules at a mind-boggling 1:1000+ ratio. Rowland and Molina found out that this specific reaction mechanism resulted in notable degradation of the ozone layer.
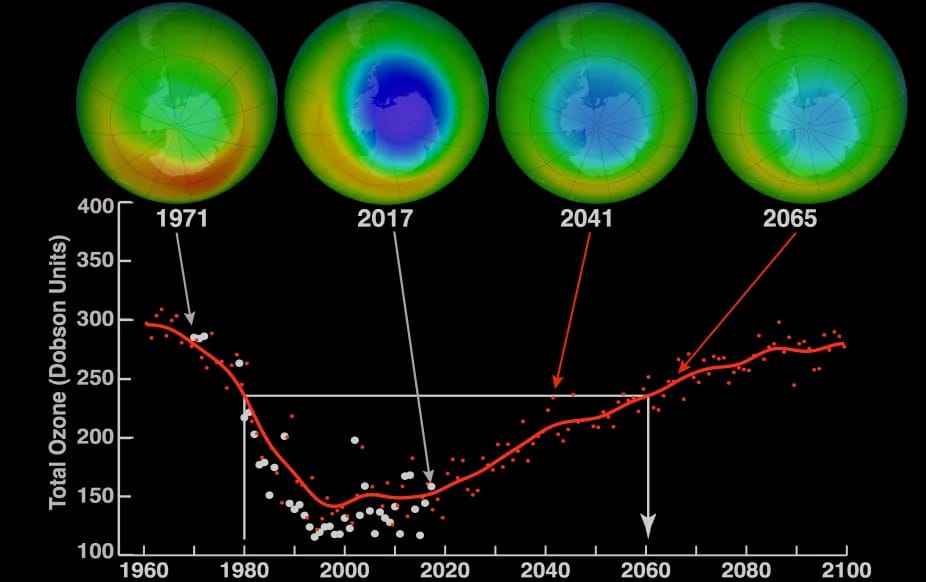
After the scientific world was alerted to the danger of CFCs, the National Academies of Science released a study in 1976 affirming Rowland and Molina's work. However, the full scale of the damage was not known until the discovery of the Antarctic ozone hole in 1985. Humanity realized that if this trend continued, the ozone hole would keep growing and potentially start harming humans en masse, so to avert this possible crisis scenario The Montreal Protocol was signed in 1987 by 56 countries that were all major CFC producers. The protocol was originally supposed to cut CFC production in half but was strengthened to cut production further.
At its peak, the ozone hole covered an area 7 times larger than the size of Europe, around 29.9 million km2, and was rapidly expanding but the effort put into cutting CFC production has shown major promise in reversing this trend and currently, the ozone hole is 26 million km2. The reduction in the size of the hole is slow and will take decades at the minimum due to the long life of CFCs in the atmosphere.
The other notable event that was occurring at the same time as humanity was realizing the damage caused by CFCs was the development of HFCs (Hydrofluorocarbons) as a safer alternative. The main distinction between CFCs and HFCs is that CFCs can last in the atmosphere for centuries whereas HFCs last for much shorter (around 1-2 decades) thus minimizing atmospheric damage. At a molecular level, they lack a chlorine atom which was the main vector through which CFCs caused ozone depletion. HFCs break down more easily as well and do not produce free radicals but this is not to say that HFCs have zero environmental impact, they have an immense impact on global warming and some HFCs have thousands of times more impact on the warming of the earth on a per gram basis than carbon dioxide. HFCs are also currently in the process of being phased out, and multiple protocols such as the Kyoto Protocol, signed by 84 signatories in 1992 and the Kagali Amendment made to the Montreal Protocol in 2016 (effective 2019, signed by 152 separate parties) include regulatory provisions on the production of HFC’s and during the COVID-19 pandemic, the United States COVID-19 relief legislation was passed which contains provisions that force chemical manufactures to cut down on HFC production.
Currently under development as an alternative to HFCs are Hydroflourooliefens (HFOs), the 4th generation refrigerants, which are unsaturated organic compounds consisting of hydrogen, carbon and fluorine. Their general characteristics are being ozone-safe (causing no damage to the ozone layer) and having low to no global warming potential. Currently, it is projected that they will, once fully developed, replace all previous-generation refrigerants.
In conclusion, CFCs were originally invented to solve a problem with toxic refrigerants and propellants, originally thought to be safe, they were found to be harmful to the ozone layer in the 80’s. Humanity was forced to face this problem and cut down its production, at the same time they were developed and adopted at a commercial scale, while HFCs and HCFCs are safer, they pose a high level of risk when it comes to global warming so multiple worldwide legislations have been signed to phase down and eventually end HFC production as well.
Similar Post You May Like
-

CFCs, HFCs and their long, troubled history
At its peak, the ozone hole covered an area 7 times larger than the size of Europe, around 29.9 million km2, and was rapidly expanding
-

The Origin of Universe: Deciding point where it all began!
Let us unravel and surf through the ideas throughout ages to understand what the universe and its origin itself was to its inhabitants across history.
-

The Artemis Program
Inspired by the Greek goddess of the Moon, twin sister to Apollo, the artimis program was named on 14 May 2019 by Jim Bridenstine.