Understanding the Quantum Theory: The photoelectric effect

It comes as no surprise that quantum physics is one of the most challenging and interesting parts of physics. This complexity arises from various foundational concepts, each building upon the other. Two crucial aspects, intimately related to the quantum revolution, are blackbody radiation and the ultraviolet catastrophe.
Blackbody Radiation:
Blackbody radiation refers to the electromagnetic radiation emitted by a perfect absorber and emitter of radiation known as a blackbody. Max Planck made a groundbreaking contribution to our understanding of blackbody radiation in 1900. Planck proposed a formula, now known as Planck's law, which successfully described the observed spectral distribution of blackbody radiation. In doing so, Planck introduced the concept of quantized energy, challenging classical physics and laying the foundation for quantum theory.
Key aspects of blackbody radiation include its spectral distribution, accurately described by Planck's law, and the quantization of energy levels, which became a fundamental principle in quantum physics.
Ultraviolet Catastrophe:
The ultraviolet catastrophe was a theoretical problem in classical physics related to the prediction of blackbody radiation. Classical physics, using the Rayleigh-Jeans law, predicted that the energy radiated by a blackbody would increase without bound as the frequency approached infinity, leading to an infinite amount of emitted energy. This contradicted observations and became known as the ultraviolet catastrophe.
Max Planck's resolution of the ultraviolet catastrophe was a landmark moment in the development of quantum theory. By introducing the quantization of energy levels, Planck prevented the predicted infinite increase in energy at extremely high frequencies, such as ultraviolet. This not only addressed the ultraviolet catastrophe but also set the stage for the quantum revolution.
Now, delving into the historical order of concepts, the exploration of wave-particle duality is crucial. This principle elucidates that light can exist as both a wave and a particle. Understanding the nature of photons, which are quantized packets of energy, forms the basis of light.
The photoelectric effect serves as a pivotal demonstration of the quantized nature of light. It involves the release of electrons when light of a specific frequency interacts with a material, highlighting key facts such as the energy of a photon being linked to its frequency, metal-specific thresholds for electron release, and the conversion of additional photon energy into the kinetic energy of released electrons.
To delve into practical applications, the observed outcomes of these quantum phenomena have practical uses, such as in photovoltaic cells for solar panels. The energy packets from sunlight result in the release of electrons from a metal plate, generating electricity. Additionally, photomultiplier tubes in imaging devices leverage the quantized nature of light to efficiently detect and convert light waves into electrical signals, with applications in medical imaging.
In conclusion, the discovery of the photoelectric effect, preceded by foundational concepts in quantum physics, has played a crucial role in shaping our understanding of the quantum nature of light. It has not only resolved debates about the wave-particle duality of light but has also paved the way for the development of quantum physics, emphasizing the quantized properties of particles such as photons. The journey from blackbody radiation to the resolution of the ultraviolet catastrophe marks a transformative period in the history of physics, heralding the quantum revolution.
Similar Post You May Like
-
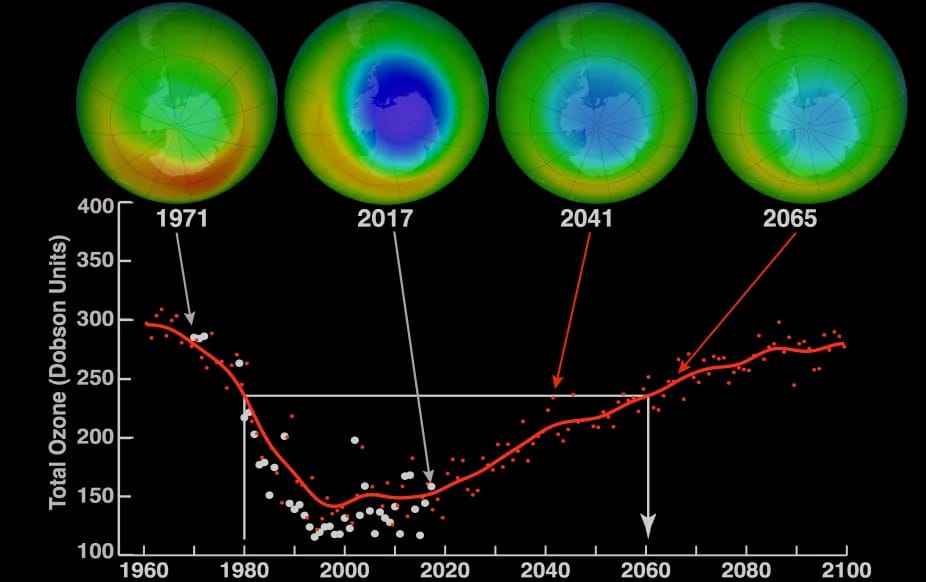
CFCs, HFCs and their long, troubled history
At its peak, the ozone hole covered an area 7 times larger than the size of Europe, around 29.9 million km2, and was rapidly expanding
-

The Origin of Universe: Deciding point where it all began!
Let us unravel and surf through the ideas throughout ages to understand what the universe and its origin itself was to its inhabitants across history.
-

The Artemis Program
Inspired by the Greek goddess of the Moon, twin sister to Apollo, the artimis program was named on 14 May 2019 by Jim Bridenstine.